




An Overview of Class 12 Chemistry To Prepare A Colloidal Solution Of Gum Experiment
Colloidal solutions are heterogeneous solutions wherein the solute particles remain dispersed in the solvent medium. The solutes in colloidal solutions are called the dispersed phase, and the solvent is known as the dispersion medium. Depending on the affinity of the dispersed phase with the dispersion medium, colloids can be lyophilic- showing high affinity towards the solvent, or can be lyophobic- showing low affinity towards the solvent. Colloidal solution examples include starch, gums, egg albumin, etc.
Table of Contents
Aim
Apparatus Required
Theory
Procedure
Observations
Results
Aim
To prepare a colloidal solution of gum.
Apparatus Required
Beakers
Glass rod
Funnel
Filter-paper
Mortar and pestle
Burner
Soluble gum
Distilled water
Theory
Lyophilic sols are solutions where the solute particles have more affinity towards the solvent and hence are more stable than lyophobic sols.
In lyophilic sols, the stability is due to the ability of colloidal particles to get dissolved in the solvent/ dispersion medium.
Charges on various colloidal particles help them to remain suspended in the solution and prevent coagulation.
The preparation of colloids, such as gum, starch etc., is accelerated if the water is hot or boiling.
Procedure
Take 500 mg of gum in a mortar and pestle and add a few ml of warm distilled water.
Grind the gum particles and make a paste of it.
Transfer this paste into a 50ml beaker.
In a 250 ml beaker, take 100 ml of distilled water and place it over a Bunsen burner.
Heat the water until it is warm.
Pour the gum paste into the 250ml beaker while constantly stirring.
Keep it in the hot water solution for a while and then allow it to cool.
Filter the beaker's contents through the filter paper using a funnel.
Note the observation and write the results.
Observations
A yellowish-coloured colloidal solution is obtained, containing particles of gum. The dispersion medium here is water, the dispersed phase is gum particles.
Result
A yellowish-coloured colloidal solution is prepared. The gum particles show a very high affinity towards the dispersion medium; hence, it is known as a lyophilic colloidal sol. It is quite stable.
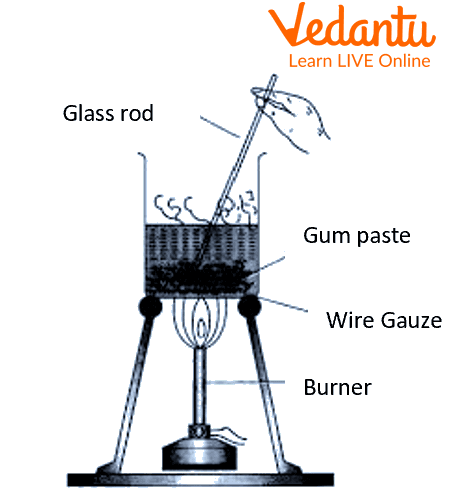
Preparation of colloidal solution of gum
Precautions
Clean all apparatus.
Distilled water has to be used and not tap water.
Stir the ingredients while pouring the paste into hot water.
Be careful while working close to the burner.
Devise precautions while pouring the paste into hot water.
Filter the gum sol carefully.
Lab Manual Questions
1. Why do particles of the dispersed phase remain suspended in the dispersed mediums?
The dispersed phase particles are either very large molecules or are made from aggregates of small molecules; hence, dispersed phase particles remain suspended in the dispersed mediums of colloidal solutions.
2. What are colloidal solutions?
Colloidal solutions are heterogeneous systems in which the solute and solvent are immiscible within each other, and hence the solute remains suspended with the solvent medium. Examples of colloids are gum sol, starch sol, and emulsifiers such as milk, butter etc.
3. What is gum?
Gums are exudates obtained from barks and various trees or shrubs. They are obtained by cutting the bark of the plants and collected every season. Gums are mostly used as adhesive material.
4. Why do we have to pour the gum paste slowly in the above experiment?
If the gum paste is poured quickly in water, it may form lumps or precipitation of the gum particles. Therefore, the prepared gum paste must be slowly poured inside the beaker containing water accompanied by constant stirring.
Viva questions
1. What is the approximate range of size of the dispersed phase particles?
Ans: The dispersed phase particle size ranges from 10-9 to 10-6 m.
2. If water is the dispersion medium, which terms are used for solvent loving and repelling?
Ans: If water is the dispersion medium, it is known as hydrophilic and hydrophobic.
3. Give examples of lyophilic sols.
Ans: Lyophilic sols are Egg albumin, starch, gums, gelatin, rubber etc.
4. What are lyophobic sols?
Ans: Lyophobic sols are solvent-repelling particles which do not have high affinity towards the dispersion medium and are very unstable. They coagulate very easily upon the addition of any electrolyte.
5. Why is gum solution prepared in warm water?
Ans: Gum gets easily dissolved in water, hence boiling water is not required, and warm water can be used.
6. Give examples of lyophobic sols.
Ans: Examples of lyophobic sols are metal sulphides, metal hydroxides, etc.
7. What is coagulation?
Ans: Coagulation is known as the process of accumulating all the colloidal particles or small aggregations of colloidal particles to settle down and form a precipitate.
8. What is peptisation?
Ans: The process through which the settled precipitate is brought back into a colloidal solution using an appropriate peptising agent is known as peptisation.
9. What are blood anticoagulants?
Ans: Anticoagulants are known as blood thinners and are chemical substances that prevent blood clots forming in our body.
10. What is hydrophilic sol?
Ans: A hydrophilic sol is a colloidal solution in which the dispersed phase has a high affinity towards water (dispersion medium).
Practical Based Questions (MCQs)
Is gum lyophilic or lyophobic?
Lyophilic
Lyophobic
Both
None of the above
Ans: Lyophilic
A type of colloidal solution where the dispersion medium and dispersed phase are liquid is known as____
Sol
Aerosol
Emulsion
Both A and C
Ans: Emulsion
What is the foam type of colloidal solution?
Gas dispersed phase in solid/liquid dispersion medium
Liquid dispersed phase in solid/liquid dispersion medium
Gas dispersion medium in solid/liquid dispersed phase
All of the above
Ans: Gas dispersed phase in solid/liquid dispersion medium
Colloidal sols which contain water-repelling solutes are known as____
Hydrophobic sols
Hydrophilic sols
Emulsified sols
Liquid sols
Ans: Hydrophobic sols
Find the odd man out.
Starch
Gum
Ferric hydroxide
Gelatin
Ans: Ferric hydroxide
Negatively charged sols are______and _____
Starch, Arsenious sulphide
Starch only, Arsenious sulphide
Hydrated Ferric oxide, water
Ferric oxide, NaOH
Ans: Starch, Arsenious sulphide
Which of the following is true-
Hydrophilic sol contains alcohol as a dispersion medium
Lyophobic sols are water-loving
Cloud is an aerosol type of colloid
Whipped cream is a type of sol
Ans: Cloud is an aerosol type of colloid
Find the odd man out
Shaving cream
Milk
Butter
Mayonnaise
Ans: Shaving cream
Why is smoke called a colloid?
Because solid particles are suspended in a gas
Because liquid, particles are suspended in water
Because oils are suspended in a gas
Because of gas, particles are suspended in a solid
Ans: Because solid particles are suspended in a gas
Which of the following is not a method of preparation of colloids?
Chemical methods
Electrical disintegration
Peptization
Coagulation
Ans: Coagulation
Conclusion
From the above experiment, we can conclude that colloidal solutions are solutions which have suspended solute particles present inside the solution. Here, the solutes and solvents do not mix completely and form a single phase. Gum, Starch, Gelatin, and rubber are common examples of lyophilic sols. Sols are a type of colloidal solution in which the dispersion medium is liquid, and the dispersed phase is solid. Lyophilic sols are solvent-loving, showing a strong affinity towards the solvent they are dispersed into.
FAQs on Class 12 Chemistry To Prepare A Colloidal Solution Of Gum Experiment
1. From an exam perspective, what are the most important things to know for the experiment on preparing a colloidal solution of gum?
For your Class 12 exams, you should focus on the correct procedure, essential precautions, and understanding the nature of the prepared sol. Be prepared for viva questions about why gum forms a lyophilic sol, how to test for its colloidal nature using the Tyndall effect, and how it differs from a true solution or a suspension.
2. What type of colloid is a gum solution, and what makes it stable?
A gum solution is a lyophilic colloid, which means 'solvent-loving'. It is highly stable for two main reasons: the particles are extensively solvated (covered with water molecules), and they carry a charge (usually negative). This combination prevents the particles from clumping together and settling down.
3. What are some key precautions to mention in the exam for preparing a gum sol?
To score well, always mention these precautions:
- Use distilled water only, as the ions in tap water can destabilise the colloid.
- Add the gum powder slowly while stirring continuously to avoid lumps.
- Ensure all apparatus, like the beaker and glass rod, are perfectly clean.
- Heat the water gently and avoid vigorous boiling.
4. How can you distinguish the prepared gum sol from a simple salt solution in an exam setting?
The most important test is the Tyndall effect. When a strong beam of light is passed through the gum sol in a dark room, its path becomes visible because the colloidal particles scatter the light. In a true solution like salt water, the path of light is not visible because the particles are too small to cause scattering.
5. For a 2-mark question, how would you explain the principle behind preparing a gum sol?
The principle involves the dispersion of a lyophilic substance in a suitable medium. Gum has a strong affinity for water. When heated, the large gum molecules break down and disperse into particles of colloidal size (1-1000 nm). This direct mixing, aided by heating, results in the formation of a stable colloidal solution.
6. Why is it necessary to use hot water to prepare a colloidal solution of gum, and not cold water?
Hot water provides the required activation energy to break down the large, complex gum molecules into smaller, colloid-sized particles. It also increases the speed of dispersion throughout the solvent. In cold water, gum would dissolve very slowly and unevenly, likely forming lumps instead of a stable, uniform colloidal sol.
7. How should you answer a viva question about the charge on gum sol particles?
You should state that gum sol particles are negatively charged. This charge arises because the gum particles tend to adsorb negative ions (anions) from the water. This uniform negative charge on all particles creates electrostatic repulsion between them, which is a key factor in preventing them from aggregating and maintaining the sol's stability.
8. What are some practical applications of gum colloids that could be asked in a 1-mark question?
Some common applications you can mention are:
- As a thickening agent in the food industry, such as in ice creams and sauces.
- As a stabiliser and emulsifier in pharmaceutical preparations and cosmetics.
- In the manufacturing of adhesives and inks.






















