




An Overview of Class 12 Chemistry To Prepare A Sample Of 2 Naphthol Aniline Experiment
2-Naphthol aniline dye has the chemical formula C₁₆H₁₂N₂O, and it is also known as Phenyl-azo-naphthol. It is an orange-red dye. It is a member of a large family of azo compounds. The term "azo compound" refers to any organic chemical compound whose molecular structure includes the azo group (-N=N-). In the structure of 2 naphthol aniline dye, The atomic groups linked to the nitrogen atoms may belong to any organic class. Still, the commercially significant azo compounds that account for more than half of the commercial dyes have the benzene group or its derivatives as the attached groups (aromatic azo compounds).

Orange-Red colour of 2-Naphthol Aniline Dye
Structure of β Beta-Naphthol
It is a fluorescent colourless (or in some cases yellow) crystalline solid. The Melting Point of Beta-Naphthol lies between 120-122 °C. The Structure of β Beta-Naphthol C₁₀H₇OH differs from 1-naphthol where the hydroxyl group is located on the naphthalene ring. In comparison to phenol, the naphthols are more reactive naphthalene homologues. Simple alcohols, ethers, and chloroform all allow the solubility of both isomers. It is also used in the preparation of phenol and the synthesis of dyes and other chemicals. 2-naphthol is a commonly used intermediate in such types of reaction.
Structure of β Beta-Naphthol
Table of Content
Aim of the Experiment
Apparatus Required
Chemicals Required
Theory
Procedure
Observation
Result
Precaution
Lab Manual Questions
Viva Questions
Practical Based Questions
Aim of the Experiment
To prepare the organic compound Beta- Naphthol Aniline from Aniline.
Apparatus Required
A 100 mL Conical Flask
A 100 mL Beaker
250 mL Beaker.
Water Pump
An Ice Bath
A Glass Rod
Funnel
Filter Paper
Chemical Required
Aniline
Sodium nitrite (NaNO₂)
2-Naphthol
Conc. Hydrochloric acid (HCl)
Glacial acetic acid
Theory
The preparation of 2-Naphthol aniline dye is carried outusing a coupling reaction. Instead of a straightforward benzene ring, it has a -OH group connected to a naphthalene molecule. Two rings of benzene are fused to form naphthalene.
Aniline and sodium nitrite combine with hydrochloric acid to produce benzene diazonium chloride. Benzene diazonium chloride further interacts with 2-naphthol to give a deep orange-coloured 2-naphthol aniline dye.
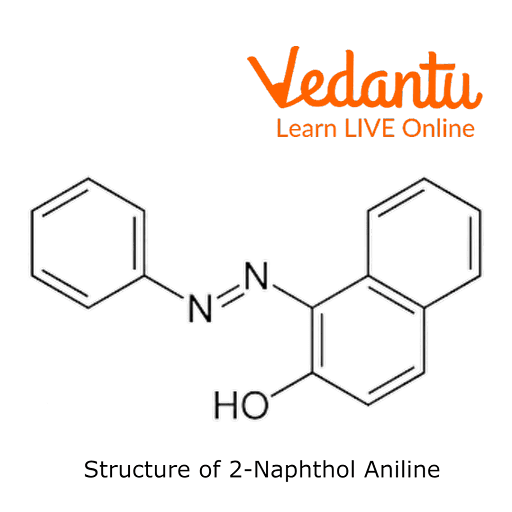
Structure of 2-Naphthol Aniline Dye
Procedure
Add 4.5 ml of aniline, 10 ml of conc. HCl, and 20 ml of water to a 100 ml conical flask. Put the conical flask in a bath of ice-cold water to cool the solution to 5°C.
Dissolve 4 g of sodium nitrite in 20 ml of water in a 100 ml beaker, and then cool this mixture to 5°C.
Now, gradually add sodium nitrite solution to the aniline solution in the cone.
In a 250 ml beaker, dissolve 7.0 g of 2-naphthol with 60 ml of a 10% NaOH solution. Cool this mixture to 5°C in an ice bath. To facilitate cooling, a little crushed ice may be directly added.
Now, slowly add the diazotised solution to the naphthol solution while stirring continuously. The phenyl-azo-naphthol quickly separates into orange-red crystals, and the mixed solutions turn red at once.
Allow the mixture to stand in the ice-salt combination for 30 minutes after the diazo solution has been added, stirring every so often. Under pump suction, filter the mixture using a funnel.
After thoroughly cleaning the phenyl-azo-naphthol with water, dry the crystals obtained by pressing them between the layers of filter paper.
Re-crystallize the end product from glacial acetic acid. Filter the crystals you got from the pump.
To get rid of acetic acid, wash with a few ml of ethanol. Orange-red crystals of phenyl-azo-naphthol can be produced. With a melting point of 133°C, a 3 g of product is expected.
Observations
Result
Phenyl-azo-naphthol obtained as orange-red crystals weigh 3 gms.
The melting point of phenyl-azo-naphthalene is 133°C.
Precautions
Due to the exothermic nature of the reaction, adding sodium nitrite should be done very gradually.
Aniline hydrochloride solution should be cooled to 5 °C, and this temperature should be maintained while adding sodium nitrite solution.
Avoid touching the concentrated acids if possible to avoid irritation.
The pH should be kept between 4-5.
Lab Manual Questions
1. Why is aniline soluble in hydrochloric acid while it is insoluble in water?
Ans. Due to the presence of highly hydrophobic benzene, aniline does not form hydrogen bonds. Aniline is thus insoluble in water. The amine group in HCl undergoes protonation (-NH₃⁺) and becomes ionic, making it soluble in HCl.
2. Why are the crystals of 2-naphthol obtained washed with ethanol?
Ans. To remove excess Acetic Acid, we wash with a few ml of ethanol. Orange-red crystals of phenyl-azo-naphthol.
3. What do you mean by coupling reaction?
Ans. Coupling reaction refers to the reaction of the diazonium salt with phenols and aromatic amines to produce azo compounds with the general formula Ar−N=N−Ar.
4. Beta naphthol aniline is the derivative of which class of organic compound?
Ans. Beta naphthol aniline is the derivative of Azo-compounds (Naphthol) of organic compounds.
Viva Questions
1. What is the use of Beta Naphthol Aniline Dye?
Ans. It is mostly employed in the dyeing of textiles.
2. Why does the colour of aniline become reddish-brown?
Ans. Due to the +R (electron donating) effect of the NH₂ group, aniline's electron density rises, and because of this, aniline is easily oxidised in air to produce compounds that are coloured. Because of this, aniline turns coloured after spending a lot of time in the air.
3. Which is more acidic: Phenol or Naphthol?
Ans. Since the hydroxyl group is directly linked to the benzene ring, phenols are significantly more acidic than alcohols.
4. Why is the end product re-crystallised from glacial acetic acid?
Ans. Orange-red crystals of phenyl-azo-naphthol are the end product re-crystallize from glacial acetic acid.
5. What is Diazotization Reaction?
Ans. Diazotisation is the process of turning a primary aromatic amino molecule into a diazonium salt. An excess of primary aromatic amine (such as aniline) in an aqueous sodium nitrite solution is added to this mixture at a temperature below 5 °C.
Practical Based Questions
What is the chemical formula of 2- Naphthol Aniline dye?
C₁₆H₁₀N₂O
C₁₆H₁₂N₂O
C₁₆H₁₂N₃O
C₁₆H₁₁N₂O
Answer. (b)
What is the IUPAC name of 2- Naphthol Aniline?
1-Phenylazo-2-naphthol
2- Naphthol
Naphthol Azobenzene
None of the Above
Answer. (a)
What is the colour of 2- Naphthol Aniline?
Pink
Reddish-brown
Yellow
Red-Orange
Answer. (d)
4. What is the IUPAC Name of 2- Naphthol?
Naphthalen-2-ol
Naphthalen-1-ol
Naphthalen-2-al
None of the above
Answer. (a)
5. What is the Melting Point of Beta Naphthol Aniline?
122 °C
133 °C
123 °C
130 °C
Answer. (b)
6. What is the solution of concentrated nitric acid and sulphuric acid called?
Alcoholic Mixture
Nitrating Mixture
Ethanolic Mixture
None of the above
Answer. (b)
7. Which of the following compounds will produce an azo dye when diazotisation and coupling with beta-naphthol are performed?
N-Methylaniline
p-Toluidine
Benzylamine
All of the above
Answer. (b)
8. How does Beta Naphthol Aniline smell?
Pungent odour
Rotten egg odour
Sweet, floral-rose odour
Odourless
Answer. (c)
9. Is Beta Naphthol Acidic or basic?
Weakly Basic
Strongly Acidic
Weakly Acidic
Strongly Basic
Answer.(c)
10. Which functional group is present in 2- Naphthol Aniline?
Hydroxy group
Carboxylic group
Amide group
Halogen group
Answer. (a)
Conclusion
The azo-compound 2-naphthol aniline dye has a red colour. It is mostly used to colour clothing. Azo compounds are pigmented and utilised as dyes. The coupling reaction is the process used to synthesise these chemical compounds. 2-Naphthol Aniline has a melting point of 133 °C. It is weakly acidic in nature. It is significantly used in dye industries to colour textiles.
FAQs on Class 12 Chemistry To Prepare A Sample Of 2 Naphthol Aniline Experiment
1. What are the essential chemical reactions involved in preparing a sample of 2-naphthol aniline dye as per the Class 12 CBSE curriculum?
The preparation of 2-naphthol aniline dye involves two main reactions that are very important for the board practical exams:
- Step 1: Diazotization Reaction: Aniline reacts with nitrous acid (formed in-situ from NaNO₂ and conc. HCl) at a low temperature (0-5°C) to form benzene diazonium chloride. This salt is the key intermediate.
C₆H₅NH₂ + NaNO₂ + 2HCl → C₆H₅N₂⁺Cl⁻ + NaCl + 2H₂O - Step 2: Coupling Reaction: The benzene diazonium chloride then acts as an electrophile and attacks the electron-rich 2-naphthol (in an alkaline medium) to form 1-phenylazo-2-naphthol, which is an orange-red azo dye.
C₆H₅N₂⁺Cl⁻ + C₁₀H₇OH → C₆H₅-N=N-C₁₀H₆OH + HCl
2. What are the key physical properties of 2-naphthol aniline dye that are important for its identification in the lab?
For the purpose of identification in the Class 12 Chemistry practical exam, the following properties are most important:
- Colour: It is a bright orange-red dye.
- State: It is obtained as crystalline solid.
- IUPAC Name: 1-Phenylazo-2-naphthol.
- Common Name: Phenyl-azo-naphthol.
- Melting Point: The expected melting point is approximately 133°C.
3. What is a coupling reaction, and why is it significant in the synthesis of azo dyes?
A coupling reaction is an organic reaction where a diazonium salt (acting as an electrophile) reacts with an electron-rich aromatic compound, such as a phenol or an aromatic amine, to form an azo compound (Ar−N=N−Ar'). This reaction is a type of electrophilic aromatic substitution. It is highly significant because the azo group (-N=N-) acts as a chromophore, which is responsible for the intense colour of the resulting compounds, making them useful as dyes.
4. List some of the most important precautions to be taken while preparing 2-naphthol aniline dye.
For a successful and safe experiment, students must observe the following precautions:
- Temperature Control: The diazotization reaction is highly exothermic. The temperature must be strictly maintained between 0°C and 5°C using an ice bath to prevent the unstable diazonium salt from decomposing.
- Slow Addition: The sodium nitrite solution should be added very slowly and with constant stirring to control the reaction rate and temperature.
- Acid Handling: Concentrated hydrochloric acid is corrosive and should be handled with care, wearing appropriate safety gear.
- pH Maintenance: The pH for the coupling reaction should be maintained correctly (typically alkaline for phenols) to ensure the 2-naphthol is activated for the electrophilic attack.
5. Why is it absolutely crucial to maintain a low temperature (below 5°C) during the diazotization of aniline? What could be an expected but wrong result if the temperature rises?
Maintaining a low temperature is the most critical condition for this experiment. The product of diazotization, benzene diazonium chloride, is highly unstable at room temperature. If the temperature rises above 5°C, the diazonium salt will readily decompose by reacting with water to form phenol and nitrogen gas. This would significantly reduce the yield of the desired azo dye and produce an impure product.
6. Explain the specific role of each main reactant in the synthesis of 2-naphthol aniline for the board exam.
Understanding the role of each chemical is a frequently asked viva question:
- Aniline (C₆H₅NH₂): It is the primary aromatic amine that provides the benzene ring and the amino group which is converted into the diazo group (-N₂⁺).
- Sodium Nitrite (NaNO₂): It reacts with hydrochloric acid to generate nitrous acid (HNO₂) in-situ, which is the actual reagent responsible for the diazotization of aniline.
- Hydrochloric Acid (HCl): It serves two purposes: first, it provides the acidic medium needed for the formation of nitrous acid, and second, it converts aniline into its salt, anilinium chloride, making it soluble in water.
- 2-Naphthol (C₁₀H₇OH): This is the coupling reagent. In an alkaline medium (like NaOH), it forms the highly reactive phenoxide ion, which is then attacked by the benzene diazonium ion to form the final azo dye.
7. How does the preparation of 2-naphthol aniline also serve as a confirmatory test for primary aromatic amines?
The entire synthesis is a classic example of the azo dye test, which is a specific confirmatory test for primary aromatic amines like aniline. The test relies on two key steps: the amine must first undergo diazotization to form a stable diazonium salt at low temperatures, and this salt must then couple with a phenol or another amine to produce a brightly coloured azo dye. The formation of a distinct orange-red precipitate upon adding the diazotized solution to 2-naphthol confirms the presence of a primary aromatic amino group in the starting compound.
8. Aniline itself is insoluble in water. Why must it be dissolved in hydrochloric acid for the experiment to work?
This is an important conceptual question. Aniline is an organic base with a large non-polar benzene ring, making it insoluble in polar water. However, for the diazotization reaction to occur, the aniline must be in an aqueous solution to react with the aqueous nitrous acid. By adding hydrochloric acid (HCl), aniline is protonated to form anilinium chloride (C₆H₅NH₃⁺Cl⁻). This anilinium salt is ionic in nature and, therefore, readily dissolves in water, creating the necessary homogeneous solution for the reaction to proceed efficiently.






















