




An Overview of Class 12 Chemistry To Prepare Colloidal Solution Of Starch Experiment
A mixture containing two substances which are insoluble with one another such that one substance remains suspended in another substance is known as a colloidal solution. The particles which remain suspended are microscopic, and their size ranges from 1 to 1000 nm. There are four types of colloids e.g.- sol, emulsion, foam, and aerosol. A sol colloid is a type of colloidal solution in which minute solid particles are dispersed in a liquid medium. E.g.- starch in water, protoplasm, gels, blood etc. Colloidal solutions are heterogeneous, they are highly stable, and show properties such as diffusion and sedimentation.
Table of Contents
Aim
Apparatus Required
Theory
Procedure
Result
Aim
To prepare a colloidal starch solution (sol).
Apparatus required
Beakers
Glass rod
Filter paper
Mortar and pestle
Burner
Wire-gauze
Tripod Stand
Theory
Lyophilic sol is the colloidal solution, wherein water is used as a dispersion medium. Starch sol is hydrophilic and hence shows strong, attractive forces with water.
Sol formation requires heating. The process of making a colloidal starch solution (sol) water and starch are heated up to 100 degrees Celsius.
These lyophilic colloids are quite stable and remain unaffected due to the presence of any impurity.
Procedure
Weigh 500 mg of starch and put it inside a mortar and pestle.
To the mortar and pestle, add a few ml of Distilled water.
Grind the starch, make a paste, and pour it into a 50ml beaker.
All 100 ml of distilled water in 250 ml of the beaker and put it over a tripod stand, and heat the beaker.
Let the water boil, and then slowly pour the starch paste into the water, while constantly stirring the mixture.
Allow the mixture to heat for 10 mins, and then cool the beaker.
Use filter paper and a funnel to filter the contents inside the beaker.
Label the filtered content as ‘starch sol’.
Observations
A white colour colloidal solution is obtained, which has small starch particles suspended. The dispersion medium here is water, and the dispersion phase is starch particles.
Result
A white-coloured colloidal solution of starch is prepared. Since solid particles are suspended in a liquid medium, and the solid particle shows high affinity towards the liquid, the starch colloidal solution prepared is a lyophilic colloidal sol.
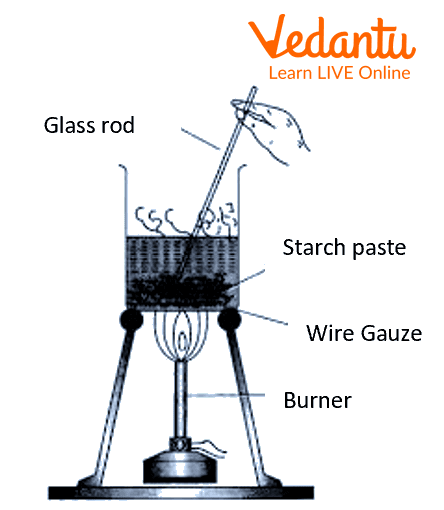
Preparation of colloidal solution of starch
Precautions
Clean the apparatus before and after use
Use proper distilled water and not tap water.
Properly grind the starch particles, or else lumps can be formed.
Put the burner's flame at the lowest while pouring the starch paste into the boiling water beaker.
Constantly stir the ingredients in the beaker.
Be careful while filtering the starch sol.
Lab Manual Questions
1. The starch solution is an example of which kind of solution?
Ans: The starch solution is an example of a hydrophilic colloidal solution. It shows a strong affinity between the dispersed phase and the dispersion medium.
2. What is sol in chemistry?
Ans: Sol colloid is a colloidal solution in which small solid particles are suspended in a liquid component. The size of the suspended solid particles is very small, and they are microscopic. E.g., Starch in water, blood, muddy water, gelatin etc.
3. What are lyophilic colloids?
Ans: Lyo means the solvent, and philic means loving or attracted to. In the presence of appropriate liquids, these colloids show high forces of attraction with them. Following are examples of lyophilic colloids-Gelatin. Gum, starch, rubber, etc.
4. What is starch?
Ans: Starch is a type of carbohydrate, a polysaccharide made of numerous glucose monomers joined by 1,4 linkages. It is white-coloured, tasteless and is used to prepare various food items.
Viva Questions
1. What is a hydrophobic colloid?
Ans: These are water-repelling or water-hating colloidal solutions. In this, the particles dispersed in water show weak attractive forces with water. For, e.g.- soil particles in river water or streams.
2. Give examples of sol in everyday food items.
Ans: Sol is a colloidal solution between a liquid and a solid. Common food items such as gravies in food, custards, jellies, gelatin etc., are all examples of sol seen in our everyday life.
3. What are emulsions?
Ans: Emulsions are colloidal solutions made from two liquids that are immiscible in each other. Here, one liquid appears as large droplets in the other liquid. E.g., Egg yolk, Butter, etc.
4. Give examples of solid and liquid aerosols.
Ans: Solid aerosols are solid particles in a gas, e.g., Smoke, dust, exhaust from cars etc. Liquid aerosols are liquid particles in a gas, e.g., Fog, mist, clouds etc.
5. Give examples of lyophobic sol.
Ans: Lyophobic sols include-Ferric hydroxide sol, Aluminium hydroxide sol, Arsenious sulphide sol, etc.
6. What are the functions of emulsifiers?
Ans: Emulsifiers help in mixing two different immiscible ingredients to get mixed up or combine. They are present in various packaged food products such as cheese, margarine, chocolate, cookies etc. These are food additives and should be taken in limited quantities.
7. What are hydrophobic particles?
Ans: Hydrophobic particles are substances with less affinity towards water, e.g.- alkanes, oils, fats, etc.
8. What is blood made of?
Ans: Blood is a colloidal solution because it contains RBCs, WBCs, and lymphocytes, which are all suspended in plasma and are made from water.
9. What is heparin?
Ans: Heparin is an anticoagulant with a high negative charge density and is produced by basophils and mast cells of the body and prevents clump-formation inside the blood.
10. Sols show which type of effect?
Ans: A sol is quite stable and shows the Tyndall effect.
Practical Based Questions (MCQs)
Find the odd man out
Sol
Emulsions
Aerosols
Solvent
Ans: Solvent
Ice cream is a type of ____
Sol
Emulsion
Aerosol
Foam
Ans: Emulsion
What prevents the coagulation of blood?
RBCs
WBCs
Heparin
Plasma
Ans: Heparin
Which of the following are colloidal solutions?
Milk, water, alcohol
Paints, Cheese
Mist, clouds, butter
Both C and B
Ans: Both C and B
A colloidal solution is prepared by____
By mixing oil and water
By adding starch in sulphuric acid
Mixing alcohol and water
By mixing salt and water
Ans: By mixing oil and water
State which of the following are false-
The starch solution, a colloid
Milk is a colloid solution
Foam colloidal solution is liquid in liquid or solid
Aerosol, contain liquid or solids present in the gas
Ans: Foam colloidal solution is liquid in liquid or solid
Which of the following are not required in starch solution preparation?
Burner
Iodine solution
Distilled water
Glass rod
Ans: Iodine solution
A sol is____
Solution containing substances
A type of colloidal solution
Contains only liquids
Is made of only gases
Ans: A type of colloidal solution
When the medium of suspension is air, then such colloidal solutions are____
Emulsions
Aerosols
Sols
Aqueous
Ans: Aerosols
A colloidal solution contains_______
Dispersed phase and Continuous medium
Solute and solvent
Salt and water
Solution and solute
Ans: Dispersed phase and Continuous medium
Conclusion
From the above experiment, we can conclude that colloidal solutions are made when two immiscible components are mixed. This causes the dispersed phase to be suspended in the continuous phase. There are 4 types of colloidal solutions as- sols, emulsions, aerosols, and foams. Various colloidal solutions are used in our daily meals, such as milk, ice-creams, butter, starch solutions, gelatin etc.
FAQs on Class 12 Chemistry To Prepare Colloidal Solution Of Starch Experiment
1. What are the essential steps to prepare a 1% colloidal solution of starch for the Class 12 Chemistry exam?
To prepare a 1% starch sol as per the CBSE curriculum for the 2025-26 session, follow these steps:
- Take about 1 gram of soluble starch in a clean beaker.
- Add a few mL of distilled water and mix thoroughly to create a thin, uniform paste. This prevents lump formation.
- In a larger beaker, bring 100 mL of distilled water to a rolling boil.
- Slowly pour the starch paste into the boiling water while continuously stirring the mixture.
- Continue to boil for another 2-3 minutes until a translucent solution is formed.
- Allow the solution to cool down. The resulting translucent mixture is the colloidal solution of starch, also known as a starch sol.
2. What are the key precautions to take while preparing a starch sol to get accurate results in the exam?
For getting clear and stable results, it's important to observe the following precautions:
- Always use distilled water, as ions in tap water can interfere with colloid formation.
- The glassware used, including the beaker and glass rod, must be thoroughly cleaned.
- The starch paste should be thin and free of lumps before being added to the boiling water.
- Add the paste slowly and with constant stirring to ensure uniform dispersion and prevent clumping.
3. Why is a starch solution classified as a lyophilic sol? This is a frequent 1-mark question.
A starch solution is called a lyophilic (solvent-loving) sol because there is a strong affinity between the dispersed phase (starch) and the dispersion medium (water). Starch molecules have numerous -OH groups that form strong hydrogen bonds with water molecules. This strong interaction makes the sol stable and relatively easy to prepare.
4. What observations confirm that a colloidal solution has been successfully formed, not a true solution?
The key observation is that the final mixture is translucent or opalescent (slightly milky), not perfectly clear like a true solution (e.g., salt in water). The most definitive test is the Tyndall effect. When a beam of light is passed through the starch sol in a dark room, the path of the light becomes visible due to the scattering of light by the larger colloid particles. A true solution does not show this effect.
5. Why is it critically important to add the starch paste to boiling water and not the other way around?
This is a crucial conceptual point for viva. Adding the starch paste to a large volume of boiling water ensures that the starch granules are dispersed quickly and evenly in the hot medium. This allows them to swell and form the colloid structure properly. If you add hot water to the paste, the heat won't be distributed evenly, causing the starch to clump together and form a lumpy, non-uniform mixture instead of a stable sol.
6. What is the specific role of continuous stirring during the preparation of a starch sol?
Continuous stirring is essential for two main reasons. Firstly, it prevents the starch particles from settling at the bottom of the beaker and forming lumps. Secondly, it ensures uniform heat distribution throughout the mixture, which helps all the starch molecules to hydrate and disperse properly to form a stable and homogeneous colloidal solution.
7. What are some expected viva questions for the Class 12 experiment on preparing a colloidal solution of starch?
Besides the procedure, an examiner might ask the following important questions:
- What is the dispersed phase and dispersion medium in this experiment?
- What is the difference between a lyophilic and a lyophobic sol?
- What is Brownian movement? Would you see it in this sol?
- How can you purify the prepared colloidal solution? (Dialysis)
- Why is the prepared sol stable?






















