




Diazonium Salts: An Overview
Amines are compounds and functional groups in chemistry, including a basic N atom with a lone pair. Amines are ammonia derivatives with one or more H atoms substituted by a substituent like an alkyl or aryl group. Diazonium salts are organic compounds with a formula $Ar-{{N_2}^{+}}-{{X}^{-}}$, where Ar is for an aromatic compound. Aryl diazonium salts are usually used as intermediates in chemical synthesis. The diazonium group is also easily replaced by a variety of functional groups, including –I, –OH, –F, –CN, and –H, that can't be directly substituted into the aromatic ring. Hence, the importance of compounds like Benzene Diazonium Chloride significantly increases in organic synthesis and in many industrial applications.

Benzene Diazonium Cation
What are Diazonium Salts?
In “Diazonium salts”, the word di refers to two, aza stands for N, and the last term onium suggests the ionic nature of the compound. As a result, diazonium salts refer to ionic compounds containing Triple bonded N atoms.
Diazonium compounds are organic compounds with the molecular formula $R-{{N_2}^{+}}-{{X}^{-}}$, where R may be any alkyl or aryl group (generally aryl groups) and X are often halogens, hydrogen sulphate, or alternative organic compounds.

Examples of Diazonium Salts
Properties of Diazonium Salt
The Physical Properties of Diazonium Compounds are as Follows:
Diazonium salts are colourless crystalline compounds that darken once exposed to air.
When heated, several diazonium salts of nitrates and perchlorates explode. As a result, these salts are not separated and are utilised in different synthetic preparations as soon as they're produced in situ.
At room temperature, however, diazonium and Zn chloride double salts, also diazonium and trifluoroborate double salts, are stable and are used as dye salts in the manufacture of naphthol-AS colouring material.
Preparation of Diazonium Salts
At 273–278K, aniline reacts with nitrous acid to get benzene diazonium chloride. Once sodium nitrite reacts with acid, nitrous acid is formed within the reaction mixture. The method of diazotization is the transformation of primary aromatic amines into diazonium ions. Because of its volatility, diazonium salt is rarely kept and is used right away when produced.

Preparation of Diazonium Cation
Benzene Diazonium Chloride
Benzenediazonium chloride could be a colourless solid and has numerous uses in chemistry. If the temperature is raised in aqueous benzene diazonium chloride, it decomposes to phenol. Therefore, benzene diazonium is prepared once it's needed for some purpose.
Benzene diazonium chloride exists in solid form.
There are no melting or boiling point values because it decomposes promptly.
Preparation of Benzene Diazonium Chloride
Benzene diazonium chloride is prepared by the reaction of aniline. Once aniline reacts with nitrous acid at low temperatures (0-50℃), benzene diazonium chloride is given as the product. If the temperature is raised, benzene diazonium chloride decomposes to phenol.

Preparation of Benzene Diazonium Chloride
Reactions of Benzene Diazonium Chloride
We can categorise reactions of benzene diazonium chloride into two categories.
Reactions of substituting diazonium group by another group
Coupling reactions of diazonium ions
The Reaction of Substituting the Diazonium Group with Another Group
Benzene diazonium chloride is often converted to different aromatic compounds like chlorobenzene, phenol, bromobenzene, etc.
Chlorobenzene may be prepared with the help of benzene diazonium chloride. Benzene diazonium chloride is treated with CuCl (Copper(I) chloride). Rather than CuCl, you can use copper powder with HCl. During this reaction, Cu+ ions behave as a catalyst. Benzene may also be prepared by benzene diazonium chloride reacting with ${{H}_{3}}P{{O}_{2}}$, ethanol as the chemical agent.
Coupling Reaction of Diazonium Ions
Benzene diazonium chloride reacts with phenol, β-naphthol, aniline, and 2-methyl aniline to form group dyes. In these reactions, the HCl molecule is eliminated.
Aniline and Benzene Diazonium Chloride Reaction
A coupling reaction between benzene diazonium chlorides with aniline in an acidic medium takes place to give a yellow colour compound. In this reaction, the nitrogen in the diazonium ion is lost and forms the bridge between two benzene rings forming an electrophilic substitution reaction. As a result, para-aminobenzene is formed as a final product.
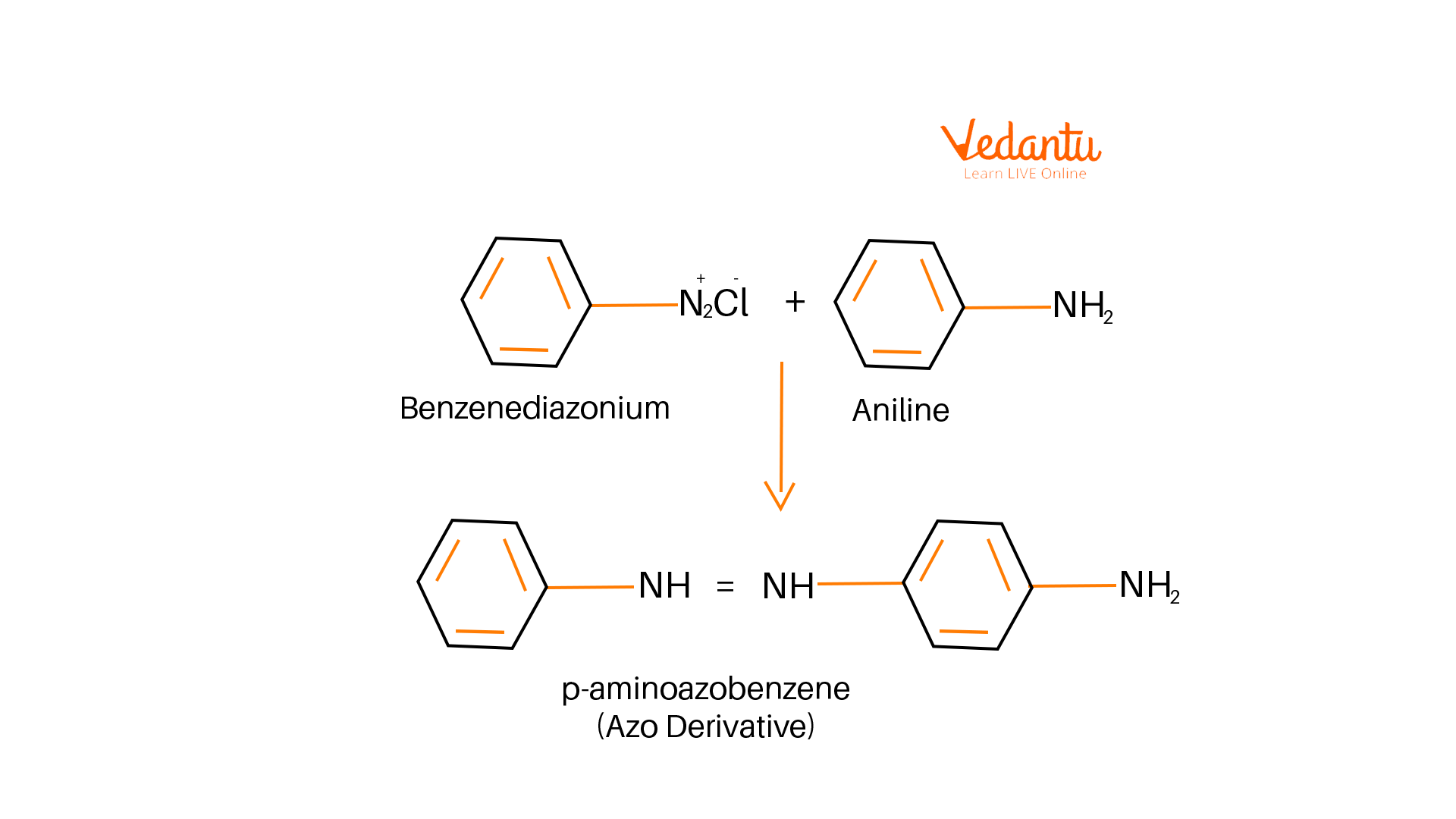
Formation of Para Amino Benzene
Importance of Diazonium Salts
Benzene diazonium chloride is used as a raw material in the production of dyes. They find application within the dye and pigment industries and are used to manufacture coloured fabrics.
Due to their property of breaking down near ultraviolet light, they're used in document reproduction, i.e., copying of papers.
They are useful in the synthesis of an outsized variety of organic compounds, particularly aryl derivatives.
Direct halogenation is not an appropriate methodology for preparing aryl iodides and fluorides. However, diazonium salts can simply be used to manufacture cyanobenzene.
It is impracticable to prepare substituted aromatic compounds by direct substitution in benzene. For these compounds, we tend to use the replacement of the diazo group in diazonium salts.
They are used as intermediates for introducing –F, –Br, –Cl, –I, –NO2, –OH, and –CN groups into the aromatic ring.
Interesting Fact
The German organic chemist Johann Peter Griess (1829–88), developed the diazotization of aryl amines.
Many diazonium salts are highly explosive and decompose violently once heated. Several diazonium salts are susceptible to displacement reactions by numerous substrates, generating nitrogen as a by-product.
Important Question
Q1. What is Sandmeyer's reaction?
Ans: $Ar-N_{2}^{+}-{{X}^{-}}\to Ar-X+{{N}_{2}}$
Cl or Br replaces the diazonium group once a freshly prepared diazonium salt solution is combined with cuprous chloride or cuprous bromide. N is slowly created at room or higher temperatures, and the aryl chloride or aryl bromide is often separated from the reaction mixture after several hours. A cuprous halide-based approach is known as the Sandmeyer reaction.
Conclusion
Diazonium salts are named by suffixing diazonium to the name of the parent organic compound (hydrocarbon) from which they're formed, followed by the name of anion like chloride, hydrogen sulphate, etc. The N2+ group is termed diazonium salt. Diazonium salts may also be used to create azo compounds by interacting with different aromatic molecules. Diazonium salts are formed by combining an alkyl or aryl primary amine with sodium nitrite in the presence of acid (HCl).
FAQs on Benzene Diazonium Chloride
1. What is Benzene Diazonium Chloride and what is its chemical formula?
Benzene Diazonium Chloride is an organic chemical compound and a type of aryl diazonium salt. It is a key intermediate in the synthesis of many aromatic compounds. Its chemical formula is C₆H₅N₂⁺Cl⁻. The 'di' refers to two, 'aza' stands for nitrogen, and 'onium' indicates its ionic nature.
2. How is Benzene Diazonium Chloride prepared according to the Class 12 syllabus?
Benzene Diazonium Chloride is prepared through a process called diazotisation. This reaction involves treating a primary aromatic amine, such as aniline, with nitrous acid (HNO₂) at a very low temperature of 273-278 K (0-5°C). The nitrous acid is generated in situ by reacting sodium nitrite (NaNO₂) with a strong acid like hydrochloric acid (HCl).
3. Why must the diazotisation reaction be carried out at a very low temperature (273-278 K)?
The diazotisation reaction must be performed at a very low temperature because Benzene Diazonium Chloride is highly unstable at higher temperatures. If the temperature rises above 5°C, the diazonium salt will readily decompose in the aqueous solution to form phenol and nitrogen gas (N₂), which is an excellent leaving group. Maintaining the low temperature ensures the stability of the diazonium salt for its use in subsequent synthetic steps.
4. What are the main physical properties of Benzene Diazonium Chloride?
Benzene Diazonium Chloride exhibits the following physical properties:
- It is a colourless crystalline solid.
- It is readily soluble in water but reacts with it when warmed.
- In its solid, dry state, it is highly unstable and can explode when heated or subjected to shock. For this reason, it is almost always prepared and used immediately in solution (in situ) without being isolated.
5. Explain the structure of the benzenediazonium ion.
The benzenediazonium ion (C₆H₅N₂⁺) consists of a benzene ring (C₆H₅) attached to a diazonium group (–N₂⁺). The two nitrogen atoms are linked by a triple bond (N≡N). One nitrogen atom is bonded to the carbon atom of the benzene ring, while the other carries a positive formal charge. This entire ion forms an ionic bond with an anion, such as chloride (Cl⁻), to form the salt. The structure's resonance stability contributes to its existence, though it remains highly reactive.
6. What are the two main types of reactions that Benzene Diazonium Chloride undergoes?
Benzene Diazonium Chloride primarily undergoes two categories of reactions:
- Displacement Reactions: In these reactions, the diazonium group (–N₂⁺Cl⁻) is completely replaced by another atom or group, such as –OH, –Cl, –Br, –CN, –I, –F, or –H. Nitrogen gas (N₂) is evolved as a byproduct. Examples include the Sandmeyer and Gattermann reactions.
- Coupling Reactions: In these reactions, the diazonium group is retained, and it couples with electron-rich aromatic compounds like phenols or anilines to form brightly coloured azo compounds. These are used extensively as dyes.
7. How does the Sandmeyer reaction differ from the Gattermann reaction for preparing aryl halides?
Both the Sandmeyer and Gattermann reactions are used to replace the diazonium group with a halide (–Cl or –Br), but they differ in the catalyst used.
- The Sandmeyer reaction uses a cuprous halide (CuCl or CuBr) dissolved in the corresponding halogen acid (HCl or HBr) as the catalyst. It generally gives a better yield.
- The Gattermann reaction is a modification that uses copper powder in the presence of the corresponding halogen acid (HCl or HBr) instead of a cuprous salt. The yield is typically lower than in the Sandmeyer reaction.
8. What is a coupling reaction? Provide an example using Benzene Diazonium Chloride.
A coupling reaction is an electrophilic substitution reaction where the diazonium ion (a weak electrophile) attacks an electron-rich aromatic ring, such as phenol or aniline. In this reaction, the nitrogen atoms are retained and form an "azo" bridge (–N=N–) between two aromatic rings. A classic example is the reaction between Benzene Diazonium Chloride and phenol in a mildly alkaline medium, which produces p-hydroxyazobenzene, an orange-coloured dye.
9. Why is Benzene Diazonium Chloride so important for synthesising a wide variety of aromatic compounds?
Benzene Diazonium Chloride is a vital synthetic intermediate for several key reasons:
- Excellent Leaving Group: The diazonium group (N₂⁺) is one of the best leaving groups in organic chemistry because it is lost as stable, gaseous dinitrogen (N₂), which drives the reaction forward.
- Versatility: It allows for the introduction of functional groups (–F, –I, –CN, –OH) onto the benzene ring that are difficult or impossible to introduce via direct electrophilic substitution.
- Synthesis of Azo Dyes: It is the precursor for a large class of industrial dyes and pigments through coupling reactions.
10. How can Benzene Diazonium Chloride be used to prepare the following compounds: (a) Phenol and (b) Benzene?
Benzene Diazonium Chloride can be converted into these compounds as follows:
- (a) Preparation of Phenol: To prepare phenol, the aqueous solution of Benzene Diazonium Chloride is simply warmed to around 283 K or higher. The diazonium salt undergoes hydrolysis, where the –N₂⁺Cl⁻ group is replaced by an –OH group, yielding phenol and releasing nitrogen gas.
- (b) Preparation of Benzene: To prepare benzene, the diazonium salt is treated with a mild reducing agent. The most common reagents used for this reduction are hypophosphorous acid (H₃PO₂) or ethanol (C₂H₅OH). These reagents reduce the diazonium group, replacing it with a hydrogen atom.






















