




Boiling Point
The boiling point goes up when a substance is dissolved in a liquid. This is an example of a colligative property, which is a property that depends on the number of solute molecules and the number of solvent molecules but not on the type of solute.
What is the Elevation of Boiling Point?
When a solute is added to a solvent, the solvent's boiling point goes up. This is called "boiling point elevation." When a non-volatile solute is added to a solvent, the boiling point of the resulting solution is higher than that of the solvent by itself. For instance, a solution of sodium chloride (salt) and water has a higher boiling point than pure water.
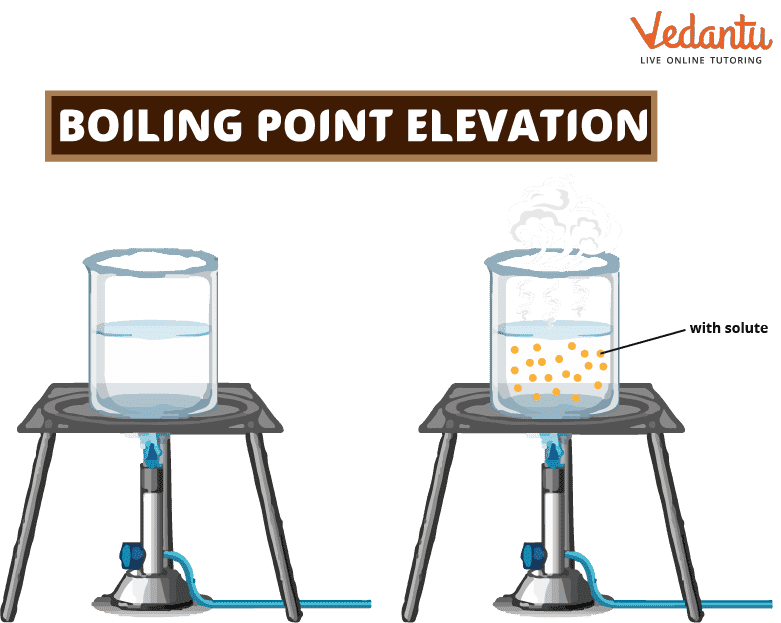
Elevation of Boiling Point
The height of the boiling point is a colligative property of matter, which means that it depends on the ratio of solute to solvent but not on what the solute is. This means that the amount of solute added to a solution changes how high its boiling point goes. The boiling point rises the higher the concentration of solute in the solution.
Why does Boiling Point Elevation Occur?
A liquid's boiling point is the temperature at which the pressure of its steam is the same as the pressure of the air around it. Non-volatile things don't quickly evaporate, and their vapor pressures are shallow (assumed to be zero). When a non-volatile solute is added to a solvent, the solution has a lower vapor pressure than the solvent.
So, the solution needs more heat to boil. The rise in the solution's boiling point is called the boiling point elevation. When the added solute increases, the solution's boiling point goes up, and its vapor pressure goes down.
Uses of Boiling Point Elevation
As we know that it doesn't have many applications of elevation in boiling point, but it does have a few uses in everyday life that you may have seen. Let's discuss the uses of boiling point elevation:
Antifreeze - Ethylene Glycol or Antifreeze helps prevent radiator water from freezing. You may not have noticed it also raises the fluid's boiling point. Raising the boiling point prevents boil-overs. Many antifreeze manufacturers list boil-over and freeze-up prevention.
Cooking - Adding salt to water increases its boiling point, making it hotter when it boils. Adding a few grams of salt to 10 cups of water raises the boiling point by 0.015 degrees Celsius, which won't affect your cooking. Cooking uses boiling point elevation. Contrary to misconception, salting water won't help it boil quicker. Since its boiling point has risen, it will take longer to boil.
Molar Mass - Boiling point relies on the solvent and solute concentration, not the solute. Like freezing point depression, boiling point elevation may determine a solute's molar mass. The number of solute particles must also be considered if the solution contains an electrolyte, like sodium chloride, which splits when dissolved. Chemists employ mass spectrometry to estimate the molar mass of substances; however, boiling point elevation and freezing point depression are still options.
Refinery - Once sugarcane is harvested and the juice removed, it must be processed to make crystalline sugar. The temperature at which cane juice or syrup boils depends on its sugar content. The boiling point elevation helps monitor solution saturation, which is critical for crystallization.
Boiling Point Elevation Examples in Real Life
Pressure Cookers
Cooking with Salt
Sugar Refining
Antifreeze
Boiling Milk
Storage of Chemicals
Poor Cup of Tea at Mountains
High Altitude Cooking
Conclusion
The boiling point rises when a solute is added to a solvent. Adding a non-volatile solute to a solvent raises the boiling point of the solution. Sodium chloride solution and water boil higher than pure water. Boiling point elevation is a colligative feature of matter, meaning it relies on the solvent-to-solvent ratio but not the solute. Adding more solution raises a solution's boiling point. Higher solute concentration raises the boiling point.
FAQs on Boiling Point Elevation
1. What is meant by the elevation of boiling point in chemistry?
Elevation of boiling point is a colligative property observed when a non-volatile solute is added to a pure solvent. It is defined as the increase in the boiling point of the solvent. The magnitude of this increase depends on the concentration of solute particles, not their chemical identity, and is a direct result of the lowering of the solvent's vapour pressure.
2. Can you explain the concept of boiling point elevation with a simple, real-world example?
A common example of boiling point elevation is adding salt (a non-volatile solute) to water (a solvent) when cooking. The salt dissolves but does not evaporate, lowering the water's vapour pressure. To make this salt water boil, it must be heated to a temperature higher than 100°C (at standard pressure). This is because more energy is needed to make its vapour pressure equal to the surrounding atmospheric pressure.
3. What is the formula used to calculate the elevation of boiling point?
The formula to calculate the elevation of boiling point (ΔTb) is: ΔTb = Kb × m. In this formula:
- ΔTb is the elevation in boiling point (Tboiling, solution - Tboiling, pure solvent).
- Kb is the molal elevation constant, also known as the Ebullioscopic Constant, which is specific to the solvent.
- m is the molality of the solution (moles of solute per kilogram of solvent).
4. What is the Ebullioscopic Constant (Kb) and what does it represent?
The Ebullioscopic Constant (Kb) is a proportionality constant that is unique to each solvent. It represents the theoretical increase in a solvent's boiling point when one mole of a non-volatile solute is dissolved in one kilogram (1 kg) of that solvent. For example, the Kb for water is 0.512 °C kg/mol. This constant is crucial for calculating molar masses of unknown solutes.
5. Why exactly does adding a non-volatile solute increase a solvent's boiling point?
Adding a non-volatile solute increases the boiling point because it interferes with the solvent's ability to escape into the gas phase. A liquid boils when its vapour pressure equals the external atmospheric pressure. When a solute is present, some solute particles occupy the liquid's surface, reducing the surface area available for solvent molecules to evaporate. This lowers the solution's vapour pressure. Consequently, more heat energy is required to raise this lowered vapour pressure to the level of the external pressure, resulting in a higher boiling point.
6. How is the 'elevation of boiling point' different from the change in boiling point at high altitudes?
These are two different phenomena with opposite effects:
- Elevation of Boiling Point is caused by adding a solute to a solvent at a constant external pressure. It lowers the vapour pressure and therefore increases the boiling temperature.
- Change at High Altitudes is caused by a change in external pressure. At higher altitudes, the atmospheric pressure is lower. This means a liquid needs less energy for its vapour pressure to equal the external pressure, causing it to boil at a lower temperature.
7. How does the concept of boiling point elevation relate to Raoult's Law?
The elevation of boiling point is a direct consequence of the principles described by Raoult's Law. Raoult's Law states that the relative lowering of a solvent's vapour pressure is equal to the mole fraction of the solute. Since the boiling point is elevated precisely because the vapour pressure is lowered, the mathematical formula for boiling point elevation (ΔTb = Kb × m) is derived directly from the relationships established by Raoult's Law for dilute solutions.
8. How can boiling point elevation be used to determine the molar mass of an unknown solute?
Boiling point elevation provides a practical method to find the molar mass (M₂) of an unknown substance. By experimentally measuring the increase in boiling point (ΔTb) when a known mass of the solute (w₂) is dissolved in a known mass of a solvent (w₁), we can use the following rearranged formula: M₂ = (Kb × w₂ × 1000) / (ΔTb × w₁). This technique is a fundamental application of colligative properties in chemistry.
9. What are the key differences and similarities between boiling point elevation and freezing point depression?
Both are colligative properties because they depend on the number of solute particles, not their nature. The key difference lies in their effect on phase transitions:
- Boiling Point Elevation: The solute lowers vapour pressure, making it harder for the solvent to boil. This increases the boiling point.
- Freezing Point Depression: The solute particles interfere with the formation of the solvent's solid crystal lattice, making it harder to freeze. This lowers the freezing point.









