
The number of stereocenters present in linear and cyclic structures of glucose are respectively:
(A) 4 and 5
(B) 5 and 5
(C) 4 and 4
(D) 5 and 4
Answer
174.3k+ views
Hint: Stereo Centre is nothing but a chiral center. A carbon which is attached to three or more different substituents is called a chiral center. If a molecule is optically active, that molecule should contain at least one chiral center in it.
Complete step by step answer:
* In the question it is given to find the number of stereocenters present in linear and cyclic structures of glucose.
* Sterocenter is nothing but the chiral center. If a carbon atom is attached to four different atoms are different groups then it is called stereocenter or chiral center.
* Chiral centers are optically active in nature.
* Linear structure and cyclic structures of glucose are as follows.
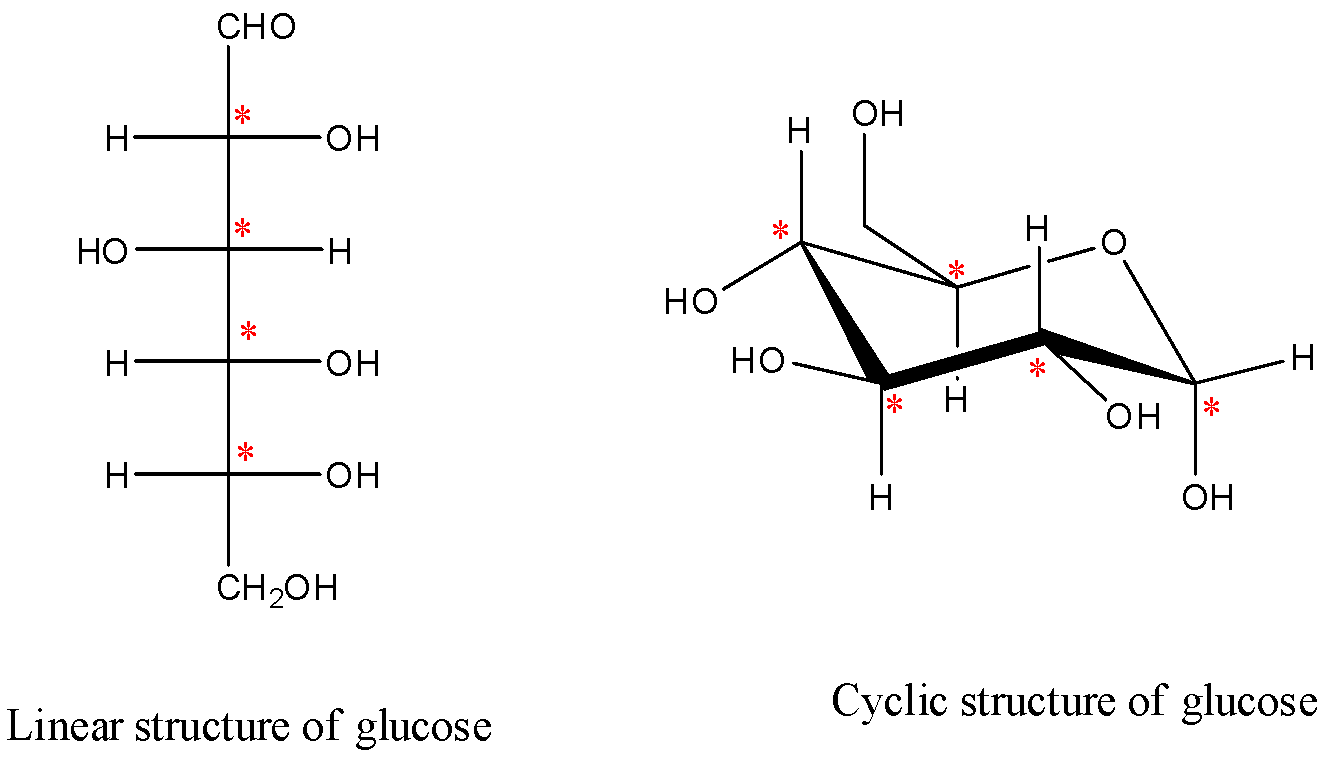
* The chiral centers are marked with red color stars.
* The number of chiral or stereo centers in linear structure of glucose are 4.
* The number of chiral or stereo centers in cyclic structure of glucose are 5.
* Therefore the number of chiral or stereo centers in linear structure and cyclic structures of glucose are 4. And 5 respectively.
So, the correct answer is A.
Additional information:
There is a reason behind one extra chiral carbon in cyclic glucose when compared to linear glucose is, after cyclisation of linear glucose one of the achiral carbon in linear structure is going to convert into chiral carbon.
Note: Linear structure of molecules is for our understanding purpose only, originally glucose exists in cyclic structures only with five chiral or stereo centers. The number of optically active isomers is also high for cyclic glucose structure when compared to linear structure of glucose.
Complete step by step answer:
* In the question it is given to find the number of stereocenters present in linear and cyclic structures of glucose.
* Sterocenter is nothing but the chiral center. If a carbon atom is attached to four different atoms are different groups then it is called stereocenter or chiral center.
* Chiral centers are optically active in nature.
* Linear structure and cyclic structures of glucose are as follows.

* The chiral centers are marked with red color stars.
* The number of chiral or stereo centers in linear structure of glucose are 4.
* The number of chiral or stereo centers in cyclic structure of glucose are 5.
* Therefore the number of chiral or stereo centers in linear structure and cyclic structures of glucose are 4. And 5 respectively.
So, the correct answer is A.
Additional information:
There is a reason behind one extra chiral carbon in cyclic glucose when compared to linear glucose is, after cyclisation of linear glucose one of the achiral carbon in linear structure is going to convert into chiral carbon.
Note: Linear structure of molecules is for our understanding purpose only, originally glucose exists in cyclic structures only with five chiral or stereo centers. The number of optically active isomers is also high for cyclic glucose structure when compared to linear structure of glucose.
Recently Updated Pages
JEE Main Hydrocarbons Mock Test 2025-26: Free Practice Online

JEE Main 2025-26 Mock Test: Organic Compounds Containing Nitrogen

JEE Main 2025-26 Mock Test: Organic Compounds Containing Halogens

JEE Main 2025-26 Biomolecules Mock Test – Free Practice Online

JEE Main 2025 Organic Compounds Containing Oxygen Mock Test

JEE Main Mock Test 2025-26: Principles & Best Practices

Trending doubts
JEE Main 2025 Session 2: Application Form (Out), Exam Dates (Released), Eligibility, & More

Displacement-Time Graph and Velocity-Time Graph for JEE

JEE Main 2025: Derivation of Equation of Trajectory in Physics

Atomic Structure - Electrons, Protons, Neutrons and Atomic Models

Learn About Angle Of Deviation In Prism: JEE Main Physics 2025

Instantaneous Velocity - Formula based Examples for JEE

Other Pages
NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 6 Haloalkanes And Haloarenes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 7 Alcohol Phenol and Ether - 2025-26




