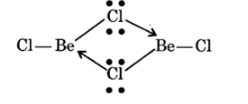
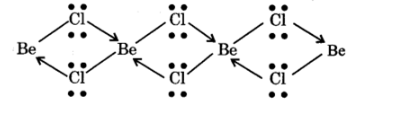
NCERT Solutions For Class 11 Chemistry Chapter 10 The S-Block Elements in Hindi - 2025-26
FAQs on NCERT Solutions For Class 11 Chemistry Chapter 10 The S-Block Elements in Hindi - 2025-26
1. Where can I find complete and step-by-step NCERT Solutions for Class 11 Chemistry Chapter 10?
Vedantu provides comprehensive NCERT Solutions for Class 11 Chemistry Chapter 10, The s-Block Elements. These solutions are crafted by subject matter experts and are fully aligned with the latest CBSE 2025-26 syllabus. Each answer is detailed, accurate, and provides a step-by-step methodology to help students understand the correct approach to solving problems.
2. Do these NCERT solutions cover all the questions from Chapter 10, including in-text and end-of-chapter exercises?
Yes, the NCERT Solutions for The s-Block Elements on Vedantu cover every single question. This includes all the in-text questions found within the chapter and the complete set of end-of-chapter exercises. Our goal is to provide a one-stop resource for all your homework and exam preparation needs for this chapter.
3. How should I structure my answers for questions comparing the properties of alkali metals and alkaline earth metals as per the NCERT solutions?
For a well-structured comparison answer, as demonstrated in our NCERT solutions, you should:
Create a table with distinct columns for the property, alkali metals (Group 1), and alkaline earth metals (Group 2).
Compare key properties like electronic configuration, atomic radii, ionisation enthalpy, and hydration enthalpy.
Provide a concluding statement summarising the main differences in their chemical reactivity.
This method ensures clarity and helps you score full marks.
4. Why do the NCERT solutions emphasize writing balanced chemical equations for the reactions of s-block elements?
The emphasis on balanced chemical equations is crucial because it aligns with the fundamental principle of the law of conservation of mass, a core concept in chemistry. For CBSE exams, correctly balancing equations demonstrates a deeper understanding of stoichiometry and reactivity. Our solutions meticulously show the balancing process to ensure you learn not just the products but also the correct mole ratios involved in the reaction.
5. What is the correct method for explaining the anomalous behaviour of Lithium in NCERT exercise questions?
According to the CBSE pattern, a complete answer explaining the anomalous behaviour of Lithium must include the following points, which are detailed in our solutions:
The exceptionally small size of the Lithium atom and its ion (Li+).
Its high polarising power, which leads to increased covalent character in its compounds.
Mentioning its high ionisation enthalpy and electronegativity compared to other alkali metals.
Citing these three reasons ensures your answer is thorough and accurate.
6. When solving NCERT problems on the diagonal relationship between Lithium and Magnesium, what key points must be included?
To solve problems on the diagonal relationship between Li and Mg effectively, your answer must highlight the similarities in their properties. The key points to include are:
Similar ionic radii (Li⁺ ≈ 76 pm, Mg²⁺ ≈ 72 pm).
Similar electronegativity and polarising power.
Mentioning specific examples, such as both forming nitrides when reacting with nitrogen and their chlorides being deliquescent, will fetch you full marks.
7. How do the NCERT Solutions for Chapter 10 explain the biological importance of sodium and potassium?
Our NCERT solutions explain the biological importance of sodium (Na⁺) and potassium (K⁺) by focusing on their specific roles in the human body. The solutions detail how:
Sodium ions are primarily found outside cells and are crucial for nerve signal transmission and regulating water balance.
Potassium ions are the most abundant cations within cell fluids, where they activate many enzymes and participate in glucose oxidation to produce ATP.
The solutions also explain the function of the sodium-potassium pump.
8. Beyond just providing the answer, how do these NCERT solutions help in understanding complex concepts like the solubility trends of s-block element salts?
Our NCERT solutions go beyond simple answers by explaining the 'why' behind the trends. For a complex topic like solubility, the solutions break down the two competing factors: lattice enthalpy and hydration enthalpy. By explaining how the balance between these two energies changes down a group or for different anions, the solutions help you build a strong conceptual foundation rather than just memorising facts. This approach is key to solving higher-order thinking (HOTS) questions.