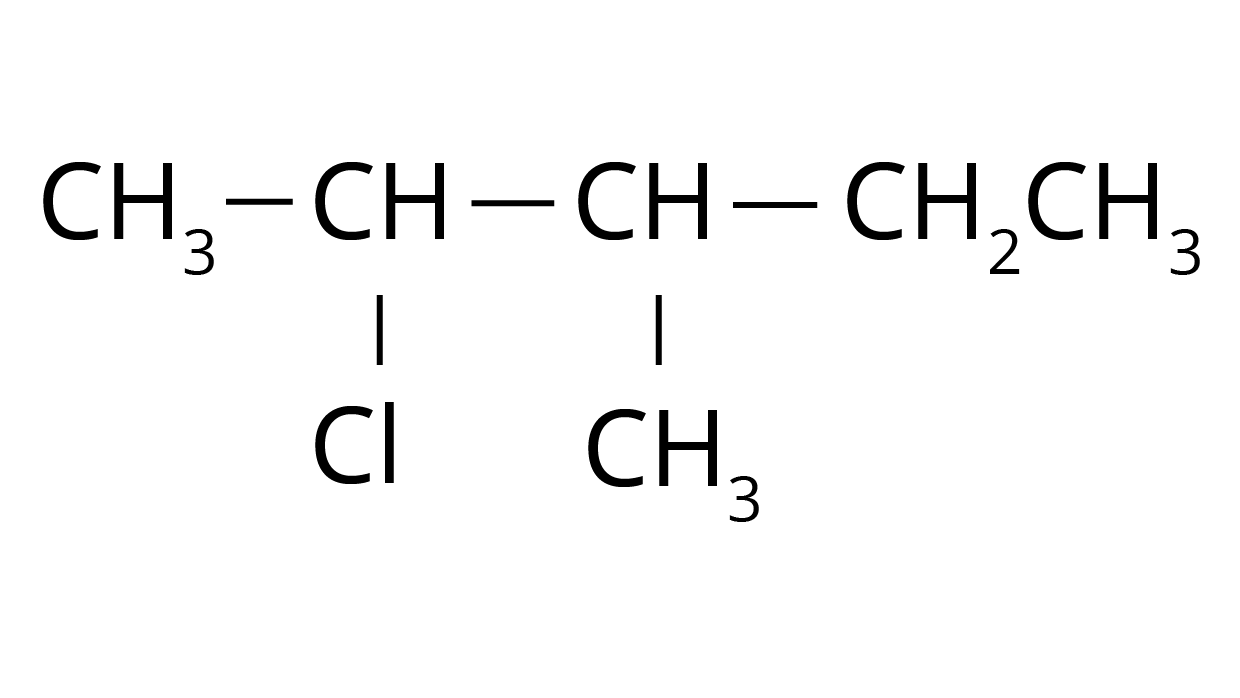NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes in Hindi Mediem
FAQs on NCERT Solutions for Class 12 Chemistry In Hindi Chapter 10 Haloalkanes and Haloarenes In Hindi Mediem
1. Where can I find the correct and updated NCERT Solutions for Class 12 Chemistry Chapter 10?
You can find reliable and detailed NCERT Solutions for Class 12 Chemistry Chapter 10, Haloalkanes and Haloarenes, fully updated for the CBSE 2025-26 syllabus on Vedantu. These solutions provide clear, step-by-step answers for all the in-text and exercise questions in the textbook.
2. How should I approach naming complex haloalkanes using IUPAC rules as per the NCERT exercises?
To correctly name haloalkanes and haloarenes as shown in the NCERT solutions, you should follow these systematic steps:
- First, identify the longest continuous carbon chain that includes the carbon atom bonded to the halogen. This is the parent chain.
- Number the parent chain starting from the end that gives the halogen-bearing carbon atom the lowest possible number.
- Name the halogen as a prefix (e.g., fluoro-, chloro-, bromo-, iodo-).
- Identify and name any other substituents (like alkyl groups), and arrange all prefixes in alphabetical order.
- Combine all parts to form the final name, ensuring correct use of numbers and hyphens, such as 2-Bromo-3-methylbutane.
3. Why do NCERT solutions show that reacting an alkyl halide with aqueous KOH gives an alcohol, while using alcoholic KOH gives an alkene?
This critical difference is based on the reaction conditions favouring either substitution or elimination. In an aqueous solution, KOH provides hydroxide ions (OH⁻), which act as strong nucleophiles, leading to a nucleophilic substitution (Sₙ) reaction to form an alcohol. In an alcoholic solution, the medium contains alkoxide ions (RO⁻), which are much stronger bases. This strong base abstracts a hydrogen atom from the β-carbon, favouring an elimination reaction to form an alkene as the major product.
4. What is the step-by-step method to determine the major alkene product in a dehydrohalogenation reaction using Saytzeff's rule?
As explained in the NCERT solutions, Saytzeff's rule states that in an elimination reaction, the more substituted (i.e., more stable) alkene is the major product. To apply this method:
- Identify the α-carbon (the one bonded to the halogen) and all adjacent β-carbons that have hydrogen atoms.
- Visualise the removal of the halogen from the α-carbon and a hydrogen atom from each unique β-carbon to form a double bond.
- Draw the structures of all possible alkene products.
- The alkene with the greatest number of alkyl groups attached directly to the double-bonded carbons is the most substituted and, therefore, the major product.
5. When solving problems on isomers, like in NCERT Exercise 10.6 for C₄H₉Br, what is a systematic way to find all possible structures?
To systematically find all isomers and avoid missing any, the NCERT solutions approach this methodically:
- First, draw all possible carbon chain isomers for the parent alkane (C₄H₁₀). In this case, they are n-butane (a straight chain) and isobutane (a branched chain).
- For each carbon skeleton, identify the chemically non-equivalent carbon atoms where the bromine (Br) atom can be attached.
- For n-butane, there are two unique positions (the end carbon and the inner carbon), which give 1-bromobutane and 2-bromobutane.
- For isobutane, there are also two unique positions (the primary carbons and the tertiary carbon), which give 1-bromo-2-methylpropane and 2-bromo-2-methylpropane.
This ensures all four structural isomers are correctly identified.
6. How do the NCERT solutions explain preparing alkyl iodides from alkyl chlorides using the Finkelstein reaction?
The Finkelstein reaction is a halogen exchange method explained in the NCERT solutions for preparing alkyl iodides. The process is as follows:
- An alkyl chloride or bromide is reacted with a solution of sodium iodide (NaI) in dry acetone.
- NaI is soluble in acetone, but the by-products, sodium chloride (NaCl) or sodium bromide (NaBr), are not.
- These insoluble by-products precipitate out of the solution. According to Le Chatelier's principle, this removal of a product shifts the equilibrium to the right, driving the reaction forward to produce a high yield of the desired alkyl iodide.
7. Why must anhydrous conditions be used when preparing a Grignard reagent, as emphasised in the NCERT textbook?
Using anhydrous (water-free) conditions is crucial because Grignard reagents are extremely reactive and are very strong bases. If any moisture is present, the Grignard reagent (R-Mg-X) will react immediately with water (a proton source) in an acid-base reaction. This would destroy the reagent by converting it into an alkane (R-H) and a magnesium hydroxy-halide (Mg(OH)X), making it unavailable for its intended synthetic purpose.
8. How do the NCERT solutions differentiate the reaction mechanisms for Sₙ1 and Sₙ2 reactions?
The NCERT solutions for Chapter 10 explain the fundamental differences between the two mechanisms:
- Sₙ2 (Bimolecular Nucleophilic Substitution): This is a concerted, single-step reaction. The incoming nucleophile attacks the substrate at the same time the leaving group departs. This mechanism is favoured by primary (1°) alkyl halides due to minimal steric hindrance.
- Sₙ1 (Unimolecular Nucleophilic Substitution): This is a two-step reaction. In the first, slow step, the leaving group departs to form a stable carbocation intermediate. In the second, fast step, the nucleophile attacks the carbocation. This mechanism is favoured by tertiary (3°) alkyl halides, as they can form the most stable carbocations.
9. In the reaction of n-BuBr with KCN, why do the NCERT solutions show n-butyl cyanide as the main product instead of the isocyanide?
The cyanide ion (CN⁻) is an ambident nucleophile, which means it can attack through either its carbon or nitrogen atom. The choice of product depends on the nature of the reagent. Since KCN is predominantly ionic, it provides free cyanide ions in solution. The attack occurs primarily through the carbon atom because the resulting C-C bond is thermodynamically more stable than a C-N bond. This leads to the formation of an alkyl cyanide (nitrile) as the major product.
10. What is the correct method for the conversion of toluene to benzyl alcohol as solved in the NCERT exercises?
The NCERT solutions outline this common conversion in two distinct steps:
- Step 1: Free Radical Halogenation: First, toluene is reacted with chlorine (Cl₂) in the presence of ultraviolet (UV) light or heat. This reaction selectively halogenates the methyl group, not the benzene ring, to form benzyl chloride (C₆H₅CH₂Cl).
- Step 2: Nucleophilic Substitution: The benzyl chloride is then hydrolysed by reacting it with an aqueous solution of KOH or NaOH. The hydroxide ion (OH⁻) displaces the chloride ion, resulting in the formation of benzyl alcohol (C₆H₅CH₂OH).