
Define catalyst.
Answer
508.5k+ views
Hint:
A catalyst is a substance that is added to a chemical reaction to increase the reaction rate without getting consumed in the process. There are basically two types of catalysts: inorganic catalysts and organic catalysts.
Complete answer:
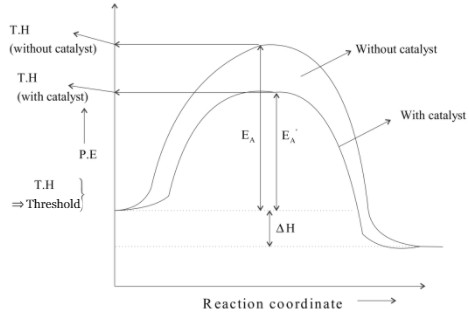
A catalyst is a substance which increases the speed of reaction without itself undergoing any chemical change. This is because the presence of a catalyst lowers the potential energy barrier and the reaction follows a new alternate path which requires less activation energy path which requires less activation energy.
Lesser is the activation energy faster is the reaction because for the energy reactant molecules can cross energy barriers and change into products.
A reaction is considered as homogeneously catalyzed when the catalyst and the reaction are in the same physical state or phase.
An example of this type of catalysis involving liquids in the conversion of percolate and iodide ions to sulfate ion and iodine,
${S_2}{O_8}^{2 - } + 2{I^ - } \to 2S{O_4}^{2 - } + {I_2}$
A reaction is considered heterogeneously catalyzed when the catalyst and the reactants are in the different phases with the reaction occurring at the interface between them.
ADDITIONAL INFORMATION:
Inorganic catalysts: Inorganic catalysts are those that are not used in biological processes. This type of catalysts includes elemental metals and some other inorganic substances. These catalysts change the rate of the reaction but do not change their structure at all. Some examples of inorganic catalysts are potassium permanganate, platinum, palladium, iron and vanadium oxide.
Organic catalysts: Organic catalysts are also known as “organocatalysts”. These catalysts include nonmetals such as carbon, sulfur, hydrogen. Like inorganic catalysts they remain in their original structure even after the reaction is complete. Some examples of organic catalysts are: proline, diastase and lactase.
Note:There are biocatalysts too. Proteins and enzymes are known as biocatalysts. The biocatalysts also fall under the category of organic catalysts. These catalysts are involved in the biological chemical processes.
A catalyst is a substance that is added to a chemical reaction to increase the reaction rate without getting consumed in the process. There are basically two types of catalysts: inorganic catalysts and organic catalysts.
Complete answer:
A catalyst is a substance which increases the speed of reaction without itself undergoing any chemical change. This is because the presence of a catalyst lowers the potential energy barrier and the reaction follows a new alternate path which requires less activation energy path which requires less activation energy.
Lesser is the activation energy faster is the reaction because for the energy reactant molecules can cross energy barriers and change into products.

A reaction is considered as homogeneously catalyzed when the catalyst and the reaction are in the same physical state or phase.
An example of this type of catalysis involving liquids in the conversion of percolate and iodide ions to sulfate ion and iodine,
${S_2}{O_8}^{2 - } + 2{I^ - } \to 2S{O_4}^{2 - } + {I_2}$
A reaction is considered heterogeneously catalyzed when the catalyst and the reactants are in the different phases with the reaction occurring at the interface between them.
ADDITIONAL INFORMATION:
Inorganic catalysts: Inorganic catalysts are those that are not used in biological processes. This type of catalysts includes elemental metals and some other inorganic substances. These catalysts change the rate of the reaction but do not change their structure at all. Some examples of inorganic catalysts are potassium permanganate, platinum, palladium, iron and vanadium oxide.
Organic catalysts: Organic catalysts are also known as “organocatalysts”. These catalysts include nonmetals such as carbon, sulfur, hydrogen. Like inorganic catalysts they remain in their original structure even after the reaction is complete. Some examples of organic catalysts are: proline, diastase and lactase.
Note:There are biocatalysts too. Proteins and enzymes are known as biocatalysts. The biocatalysts also fall under the category of organic catalysts. These catalysts are involved in the biological chemical processes.
Recently Updated Pages
Physics and Measurement Mock Test 2025 – Practice Questions & Answers

NCERT Solutions For Class 5 English Marigold - The Little Bully

NCERT Solutions For Class 12 Maths Three Dimensional Geometry Exercise 11.1

NCERT Solutions For Class 11 English Woven Words (Poem) - Ajamil And The Tigers

NCERT Solutions For Class 6 Hindi Durva - Bhaaloo

NCERT Solutions For Class 12 Physics In Hindi - Wave Optics

Trending doubts
1 ton equals to A 100 kg B 1000 kg C 10 kg D 10000 class 11 physics CBSE

Difference Between Prokaryotic Cells and Eukaryotic Cells

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE

Proton was discovered by A Thomson B Rutherford C Chadwick class 11 chemistry CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




