
The number of sigma and pi bonds in benzene are:
a. 6 sigma and 3 pi bonds
b. 12 sigma and 3 pi bonds
c. 9 sigma and 3 pi bonds
c. 6 sigma and 6 pi bonds
Answer
533.1k+ views
Hint: Benzene is a hydrocarbon composed of six carbons connected via covalent bond, and exists in the form of a ring. It has three alternating double bonds which makes the compound very stable.
Complete step by step answer:
Benzene is an aromatic compound of carbon and hydrogen. The molecular formula for benzene is \[{{C}_{6}}{{H}_{6}}\].
Let us draw the structure of Benzene.

To calculate the number of sigma bonds in this, we can draw the skeletal structure by only drawing the sigma bonds –
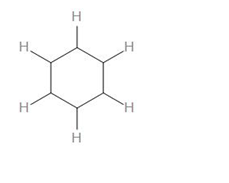
As we can see, there are 6 C-H bonds, 6 C-C bonds.
Therefore, we can say that there is a total of 12 sigma bonds in benzene.
Now, looking at the structure of benzene, we can see that there are 3 C=C bonds.
Therefore, there are 12 sigma bonds and 3 pi bonds. Benzene is therefore made up of 15 covalent bonds.
Therefore, the answer is – option (b) – The number of sigma and pi bonds in benzene are 12 and 3, respectively.
Additional Information: One single bond contains one sigma bond; a double bond contains one sigma and one pi bond.Similarly, a triple bond contains one sigma and two pi bonds.
Note: Benzene exists as a colorless and highly flammable liquid with a sweet smell. This sweet smell is produced as a result of aromaticity of the compound. Aromaticity of benzene arises due to the continuous cyclic pi bonds between the carbon atoms. It is a very stable compound. Benzene is naturally present in crude oil and is also an elementary petrochemical.
Complete step by step answer:
Benzene is an aromatic compound of carbon and hydrogen. The molecular formula for benzene is \[{{C}_{6}}{{H}_{6}}\].
Let us draw the structure of Benzene.

To calculate the number of sigma bonds in this, we can draw the skeletal structure by only drawing the sigma bonds –

As we can see, there are 6 C-H bonds, 6 C-C bonds.
Therefore, we can say that there is a total of 12 sigma bonds in benzene.
Now, looking at the structure of benzene, we can see that there are 3 C=C bonds.
Therefore, there are 12 sigma bonds and 3 pi bonds. Benzene is therefore made up of 15 covalent bonds.
Therefore, the answer is – option (b) – The number of sigma and pi bonds in benzene are 12 and 3, respectively.
Additional Information: One single bond contains one sigma bond; a double bond contains one sigma and one pi bond.Similarly, a triple bond contains one sigma and two pi bonds.
Note: Benzene exists as a colorless and highly flammable liquid with a sweet smell. This sweet smell is produced as a result of aromaticity of the compound. Aromaticity of benzene arises due to the continuous cyclic pi bonds between the carbon atoms. It is a very stable compound. Benzene is naturally present in crude oil and is also an elementary petrochemical.
Recently Updated Pages
Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

Why is the cell called the structural and functional class 12 biology CBSE

a Tabulate the differences in the characteristics of class 12 chemistry CBSE

Who discovered the cell and how class 12 biology CBSE

Pomato is a Somatic hybrid b Allopolyploid c Natural class 12 biology CBSE




