
What type of defect is shown by NaCl in:
(a) Stoichiometric defects, and
(b) Non-stoichiometric defects
Answer
501.6k+ views
Hint: The imperfection in the crystals implies the imperfect arrangement of the constituent ions is due to the presence of cationic or anionic vacancies in the lattice points or dissociation of ions in the lattice. This imperfection is known as a defect.
Complete step by step answer:
The atomic defects or point defect is the defect that occurs in ionic crystals when the number of cations and anions missing are equal or when an ion of the ionic crystals is missing and is shifted to a vacant interstitial site structure.
Point defects are found in both stoichiometric and non stoichiometric crystals which are known as stoichiometric defects and non stoichiometric defects respectively.
Both the type of point defects can be seen in NaCl,
(a) stoichiometric defect :
The Schottky defects are seen in NaCl. The defects are created when the same number of $C{l^ - }$ and $N{a^ + }$ is missing from their position in the lattice and creating holes. Even after missing some ions, it will be electrically neutral.
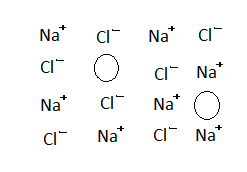
(b) Non-stoichiometric defect:
Metals excess defect produced by the presence of extra one or more cations in the interstitial site, this defect is seen in NaCl. This defect is produced when one or more $N{a^ + }$ ions occupy interstitial sites. Then, in order to maintain the crystal electrically neutral, equal number of electrons trapped into the interstitial sites.

The number of metals are excess in the crystal so the defect is known as metal excess defect.
Note: Note: NaCl is actually colourless crystal. Metals excess defect is a type of non-stoichiometric defect. Non-stoichiometric defect NaCl is yellow in colour, this is one of the consequences of metal excess defect.
Complete step by step answer:
The atomic defects or point defect is the defect that occurs in ionic crystals when the number of cations and anions missing are equal or when an ion of the ionic crystals is missing and is shifted to a vacant interstitial site structure.
Point defects are found in both stoichiometric and non stoichiometric crystals which are known as stoichiometric defects and non stoichiometric defects respectively.
Both the type of point defects can be seen in NaCl,
(a) stoichiometric defect :
The Schottky defects are seen in NaCl. The defects are created when the same number of $C{l^ - }$ and $N{a^ + }$ is missing from their position in the lattice and creating holes. Even after missing some ions, it will be electrically neutral.
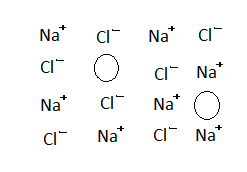
(b) Non-stoichiometric defect:
Metals excess defect produced by the presence of extra one or more cations in the interstitial site, this defect is seen in NaCl. This defect is produced when one or more $N{a^ + }$ ions occupy interstitial sites. Then, in order to maintain the crystal electrically neutral, equal number of electrons trapped into the interstitial sites.

The number of metals are excess in the crystal so the defect is known as metal excess defect.
Note: Note: NaCl is actually colourless crystal. Metals excess defect is a type of non-stoichiometric defect. Non-stoichiometric defect NaCl is yellow in colour, this is one of the consequences of metal excess defect.
Recently Updated Pages
Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

Why is the cell called the structural and functional class 12 biology CBSE

a Tabulate the differences in the characteristics of class 12 chemistry CBSE

Who discovered the cell and how class 12 biology CBSE

Pomato is a Somatic hybrid b Allopolyploid c Natural class 12 biology CBSE




